Abstract
Background: The minimally invasive technology mass spectrometry (MS) is emerging as a promising approach for detecting and monitoring monoclonal proteins in the peripheral blood of multiple myeloma (MM) patients. We aimed to define implications and prognostic value of MS in a large cohort of transplant-eligible patients with newly-diagnosed MM treated within the phase 3 multicenter GMMG-MM5 trial (EudraCT No. 2010-019173-16).
Patients and Methods: For quantitative immunoprecipitation MS (QIP-MS), we included peripheral blood serum samples from 480 patients at baseline, from 444 of these patients after 3 cycles induction therapy, from 305 patients prior to lenalidomide maintenance treatment (max. 2 years) or observation (in case of complete response [CR] in arm B of the trial), and from 227 patients after 1 year (+/- 3 months) of lenalidomide maintenance / observation. QIP-MS was carried out using the automated EXENT® assays and system (in development; The Binding Site Group Ltd., UK). Minimal residual disease (MRD) in the bone marrow was detected using an allele-specific oligonucleotide (ASO) PCR with a sensitivity of at least 1x10-6. Kaplan-Meier method was used for survival analyses. Progression-free survival (PFS) time was measured from the respective landmark to progression or death from any cause, whichever occurred first. For analyses of sustained MS from start of maintenance / observation until 1 year (+/- 3 months), PFS was calculated from the second time point (after 1 year) and only patients who did not have a prior progression event were included.
Results: MS negativity rates increased from 6% post induction to 31% and 44% prior to and after 1 year of maintenance / observation, respectively. MS negativity was associated with improved PFS at all investigated timepoints (post induction: hazard ratio [HR]=0.41; p=0.003), prior to maintenance / observation: HR=0.66, p=0.01 and after 1 year of maintenance / observation: HR=0.37, p<0.001.
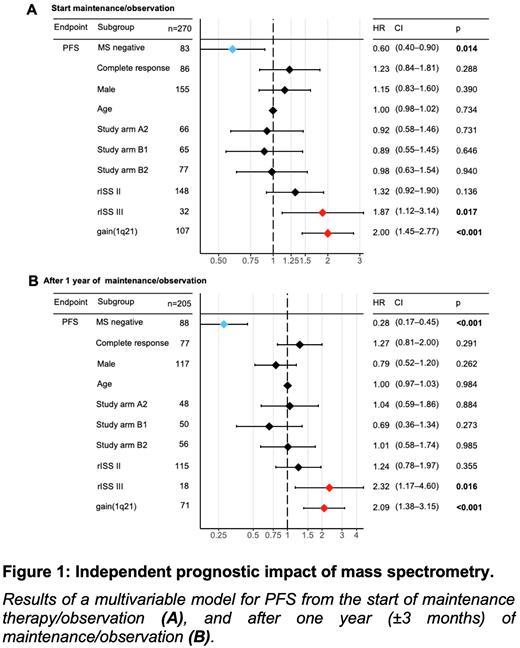
Next, we evaluated the prognostic value of MS in a multivariable model, which included age at diagnosis, gender, treatment arm, conventional response (CR vs. no CR), revised International Staging System (R-ISS) stages and gain1q21. MS negativity was an independent prognostic factor for PFS (prior to maintenance / observation: HR=0.60, p=0.01, Figure 1A; and after 1 year of maintenance / observation: HR=0.28, p<0.001, Figure 1B).
The prognostic impact of MS could be improved by baseline disease features, such as high-risk cytogenetics. Combining the FISH high-risk markers of the R-ISS del(17p13), t(4;14), and t(14;16) as well as gain1q21 with MS before maintenance / observation, patient groups with excellent or dismal outcomes could be defined. The best outcome was seen for patients without high-risk cytogenetics and MS negativity (median PFS: 4.8 years), while patients with high-risk FISH and MS positivity had a median PFS of just 1.9 years from start of maintenance / observation.
Next, we aimed to determine the prognostic value of sequential MS testing and chose the time points prior to maintenance / observation and after 1 year. The best outcome was seen for patients who converted from MS positivity to negativity (median PFS not reached). The worst outcome was seen for patients who converted from MS negativity to positivity with a median PFS of only 0.6 years. Sustained MS negativity was associated with improved PFS (median PFS: 3.5 years) as compared to sustained MS positivity (median PFS: 1.9 years, p=0.007).
In a pilot study, we combined MS with ASO-PCR for detection of MRD in the bone marrow in 45 patients with suspected CR. Double (MS / MRD) positive patients had the worst outcome (median PFS: 2.2 years) whereas double-negative patients had a median PFS of 3.3 years and no overall survival events.
Conclusions: Our study provides strong evidence that MS is superior to conventional response monitoring and should become a new standard to monitor monoclonal protein in MM. We recommend performing MS testing sequentially and combined with baseline disease features to improve prognostication. Disease relapse in MS / MRD double-negative patients in our study probably occurred due to limited duration of lenalidomide maintenance or observation, but also highlights that even a combined approach with a highly sensitive bone-marrow MRD tool was not sufficient to identify disease-free MM patients.
Disclosures
Mai:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding. Scheid:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Weisel:Amgen: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Novartis: Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria; Adaptive Biotech: Consultancy, Honoraria; AstraZeneca: Honoraria; BeiGene: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Stemline: Honoraria. Munder:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expanses. Berlanga:The Binding Site: Current Employment. Hose:LamKap Bio: Current Employment. Seckinger:LamKap Bio: Current Employment. Haenel:Takeda: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; JAZZ: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Pfizer: Honoraria. Salwender:Sanofi: Honoraria; Takeda: Honoraria; Oncopeptides: Honoraria; GSK: Honoraria; Janssen: Honoraria; BMS: Honoraria; Abbvie: Honoraria; Amgen: Honoraria. Raab:Takeda: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Heidelberg Pharma: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees. Goldschmidt:Amgen, BMS, Celgene, Chugai, Dietmar-Hopp-Foundation, Janssen, Johns Hopkins University, Sanofi: Other: Grants and/or provision of Investigational Medicinal Product; Takeda: Research Funding; Novartis: Honoraria, Research Funding; Mundipharma GmbH: Research Funding; Merck Sharp and Dohme (MSD): Research Funding; BMS: Consultancy, Honoraria, Other: Grants, Research Funding; AMGEN: Consultancy, Honoraria, Other: Grants, Research Funding; Adaptive Biotechnology: Consultancy; Celgene: Consultancy, Honoraria, Other: Grants, Research Funding; Amgen, BMS, Celgene, Chugai, Janssen, Incyte, Molecular Partners, Merck Sharp and Dohme, Sanofi, Mundipharma GmbH, Takeda, Novartis: Research Funding; Incyte: Research Funding; Molecular Partners: Research Funding; SANOFI: Consultancy, Honoraria, Other: Grants, Research Funding; Janssen: Consultancy, Honoraria, Other: Grants, Research Funding; Chugai: Honoraria, Other: grants, Research Funding; Amgen, BMS, Janssen, Sanofi, Takeda: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline (GSK): Honoraria; Amgen, BMS, Chugai, GlaxoSmithKline, Janssen, Novartis, Sanofi, Pfizer: Honoraria; Amgen, BMS, GlaxoSmithKline, Janssen, Novartis, Sanofi, Pfizer: Other: Support for attending meetings and/or travel; Array Biopharma: Research Funding; Dietmar-Hopp-Foundation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal